 Electronic Data Capture Systems
Electronic Data Capture Systems







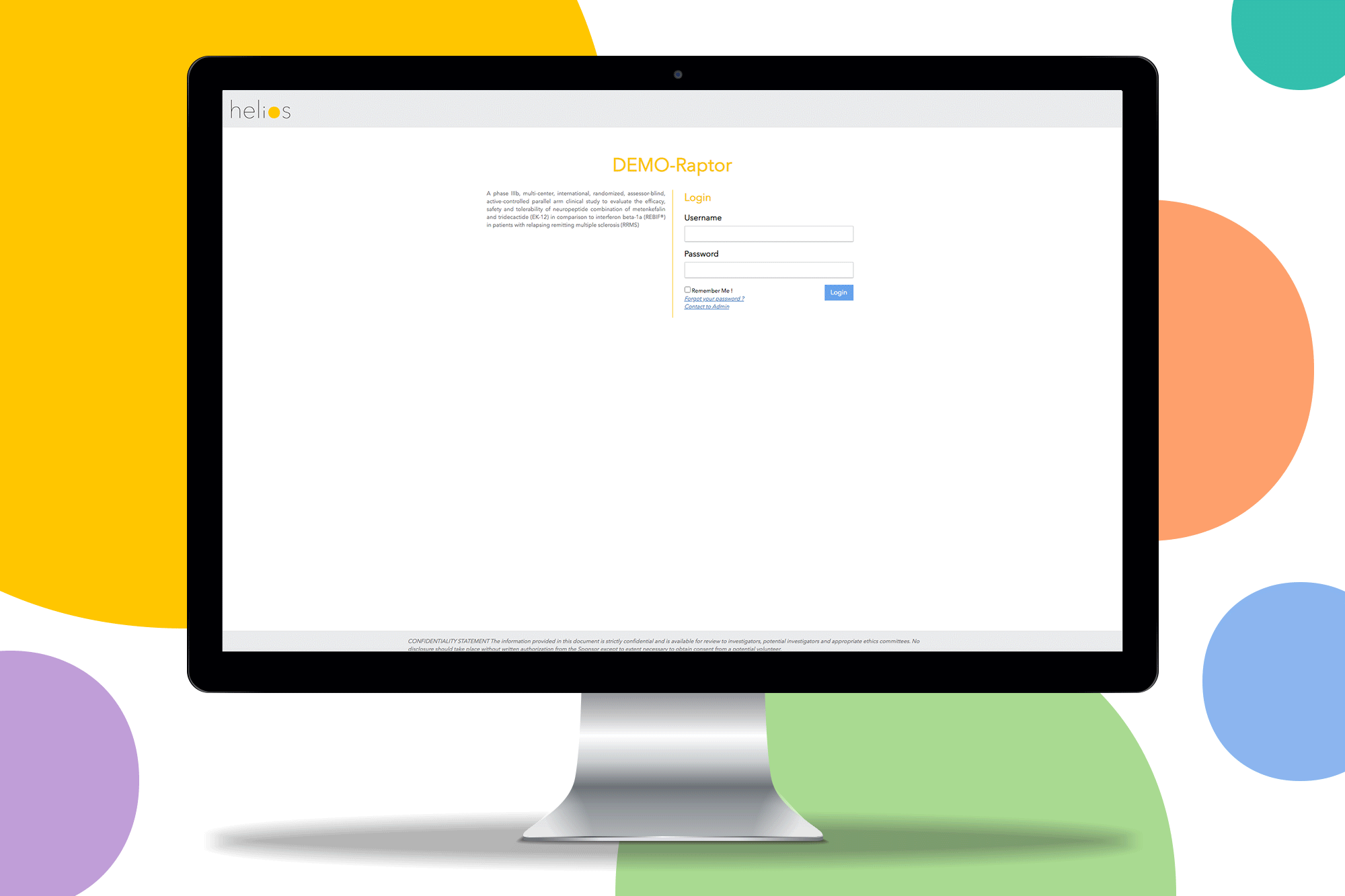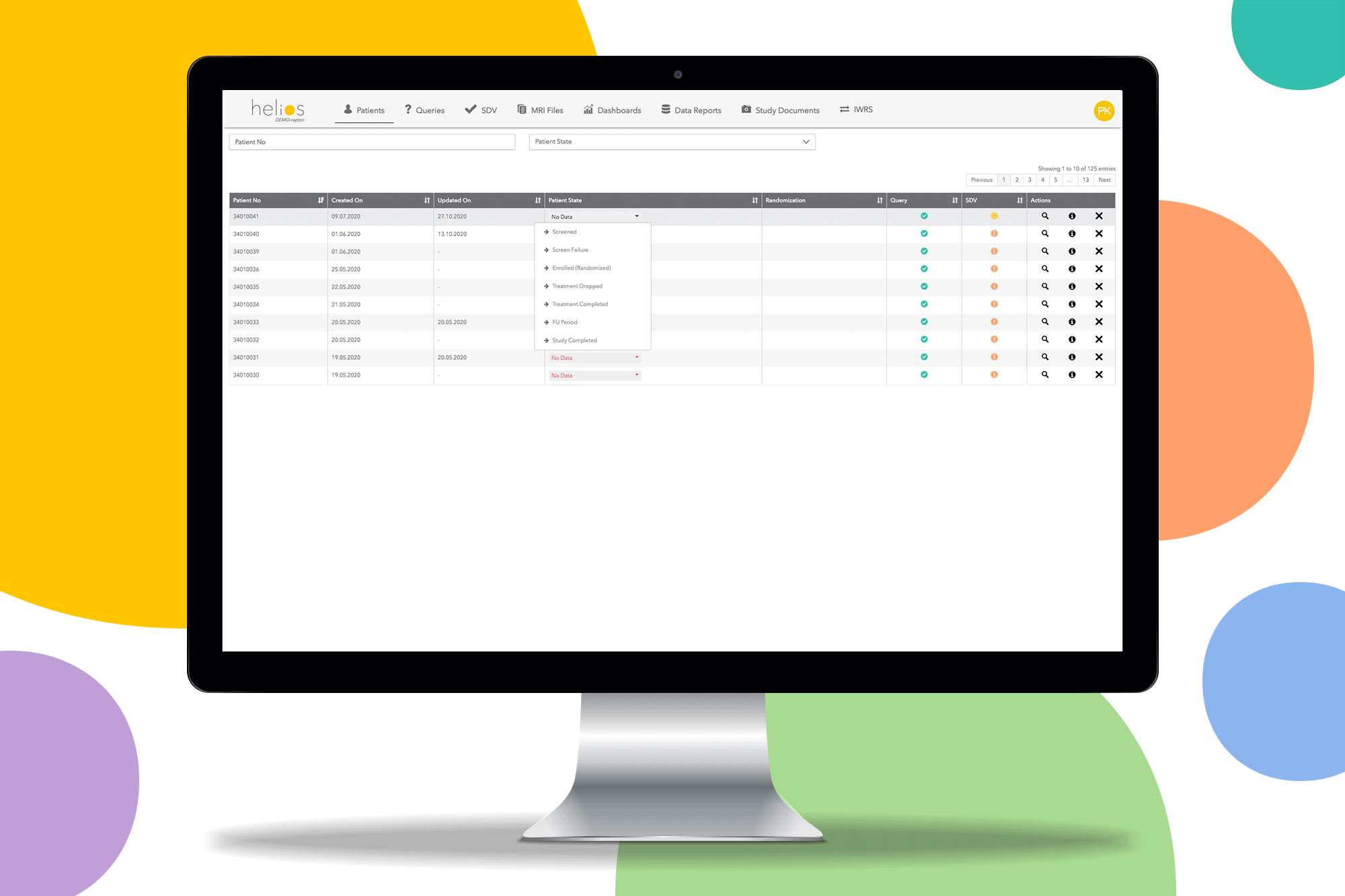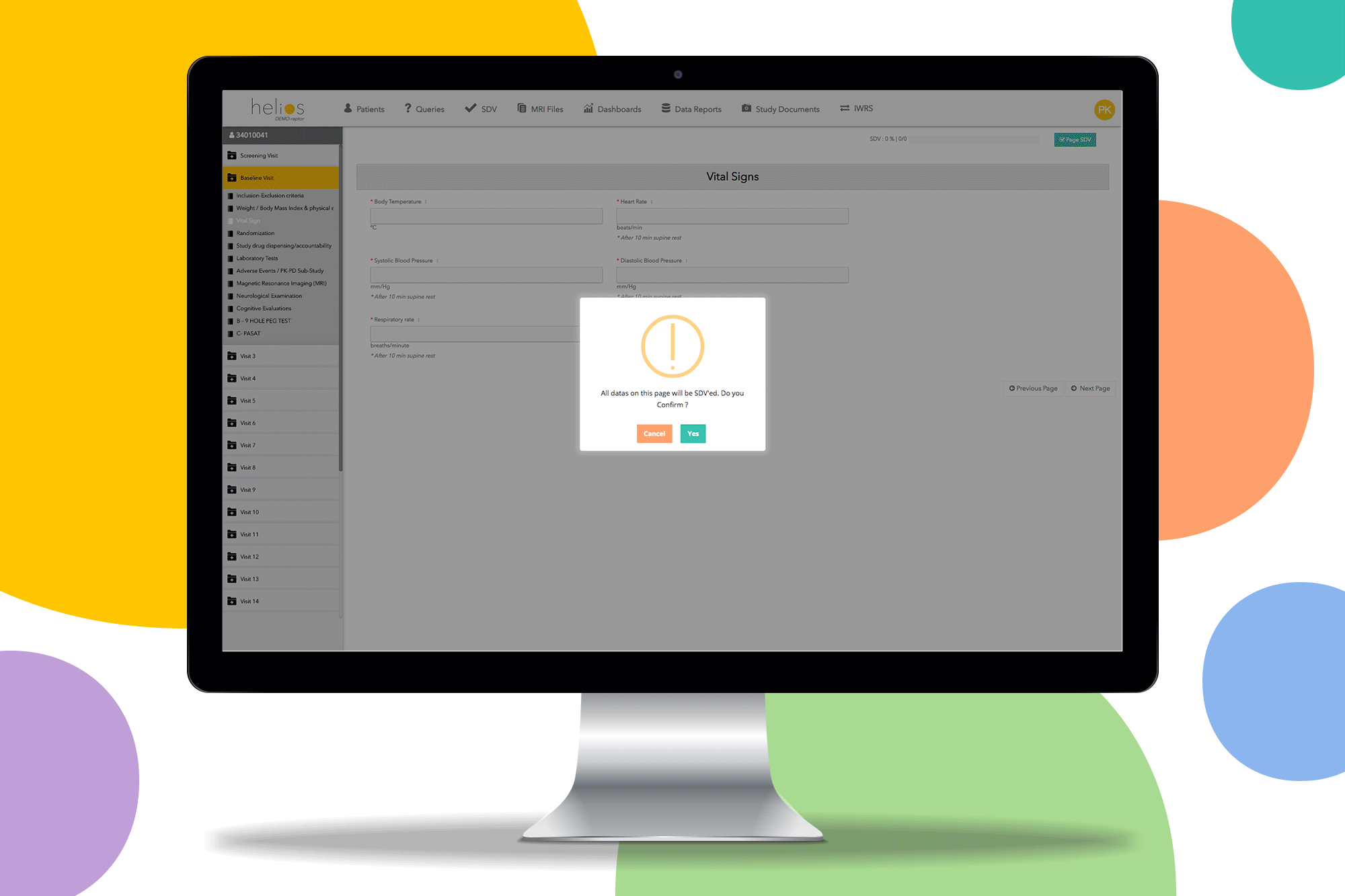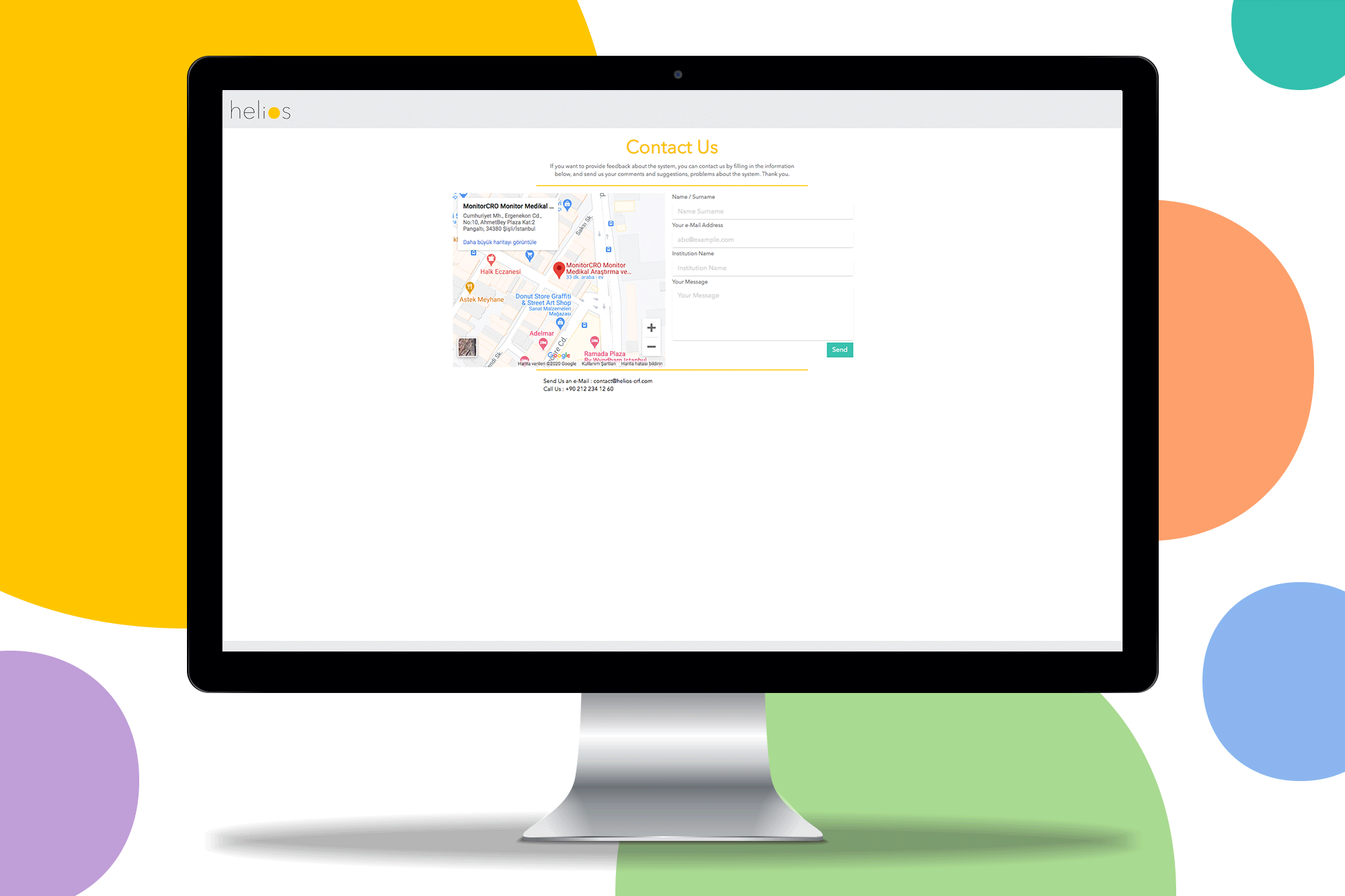
Helios EDC provides a fast and secure solution to capture and review data on sites. Remove restrictions and start the study you want with a contemporary EDC system.
Build studies quickly and efficiently with our data management team. Drag-and-drop form design, easy reuse of templates and forms. Automatic calculations and edit checks
Collect data sets from any source and harmonize them in one place, access your study data from anywhere in no time with study spesific dashboards.
Easily assign Role based permissions to project management teams and researchers in opening new sites
Built in reporting templates for missing SDV and uncompleted forms to make monitoring easy so you can only focus on data quality.
Make mid-study changes in real time with multiple data entry options and intuitive navigation
Track the progress of trial enrolment in real time with 24/7 availability of the service.
Securely randomize patients and bring your clinical trial to the next level with double blinded randomization.





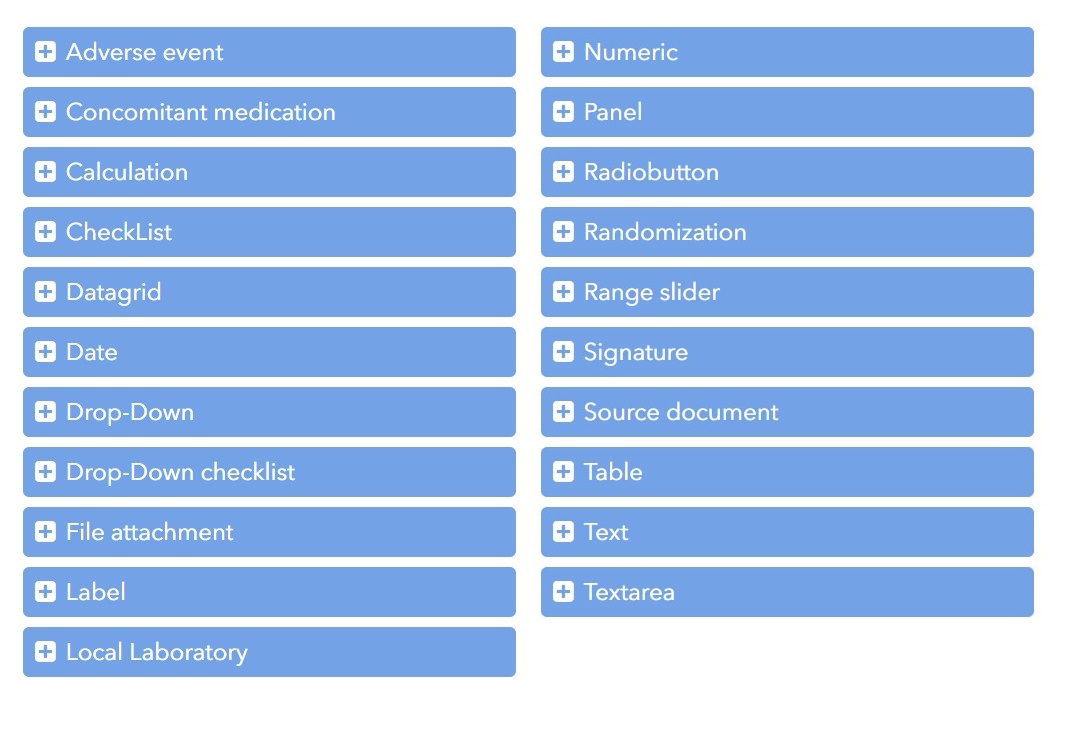
Build studies quickly and efficiently with our data management team. Drag-and-drop form design, easy reuse of templates and forms. Automatic calculations and edit checks
Collect data sets from any source and harmonize them in one place, access your study data from anywhere in no time with study spesific dashboards.

Easily assign Role based permissions to project management teams and researchers in opening new sites
Built in reporting templates for missing SDV and uncompleted forms to make monitoring easy so you can only focus on data quality.


Validation packeges for IQ-OQ procedures and study data validation documents can be created automatically for your study.
Make mid-study changes in real time with multiple data entry options and intuitive navigation


Track the progress of trial enrolment in real time with 24/7 availability of the service.
Securely randomize patients and bring your clinical trial to the next level with double blinded randomization.
